
For the undiscovered eighth-row elements, mixing of configurations is expected to be very important, and sometimes the result can no longer be well-described by a single configuration. In many cases, multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to chemical behaviour. Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. However there are numerous exceptions for example the lightest exception is chromium, which would be predicted to have the configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 4s 2, written as 3d 4 4s 2, but whose actual configuration given in the table below is 3d 5 4s 1. Carbon (atomic number 6) has six electrons. Electron configurations of elements beyond hassium (element 108) have never been measured predictions are used below.Īs an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule. In this figure, the element symbol B is followed by the electron configuration. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell.

In addition to explaining why some elements form more bonds than would be expected based on their valence electron configurations, and why the bonds formed are equal in energy, valence bond theory explains why these compounds are so stable: the amount of energy released increases with the number of bonds formed.This page shows the electron configurations of the neutral gaseous atoms in their ground states. Write the electron structure of the two cations. Along with the protons and electrons, the atom consists of neutrons as well which may or may not be in the same. Each neutral atom has a fixed number of electrons which equals the number of protons present and is called the atomic number.
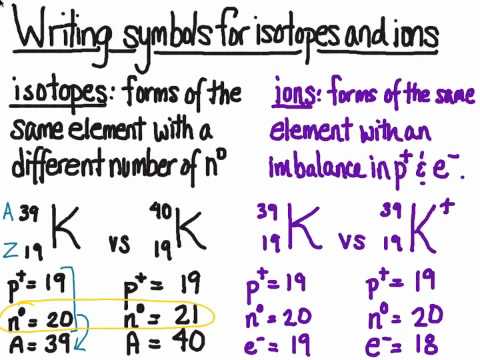
For this reason, carbon will form an excited state by promoting one of its 2s electrons into its empty 2p orbital and hybridize from the excited state. Electron configuration can be defined as the distribution of electrons across the orbitals of an atom. Lets look at the electron configuration of ground state (lowest energy state) carbon. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+. By looking at the electron configuration, one is able to identify these valence electrons. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Not always a valid charge reference value (e.g., C1s peak for adventitious carbon on native oxide of. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. The C-C component may be set to a binding energy of 284.8eV, by default. In the three dimensional figure below, the first and most inner electron shell is represented by blue electrons, the second electron. C1s spectrum for contamination typically has C-C, C-O-C, and O-CO components. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. Combining one ns and three np atomic orbitals results in four sp 3 hybrid orbitals oriented at 109.5° to one another in a tetrahedral arrangement. Adventitious carbon contamination is commonly used as a charge reference for XPS spectra.


 0 kommentar(er)
0 kommentar(er)
